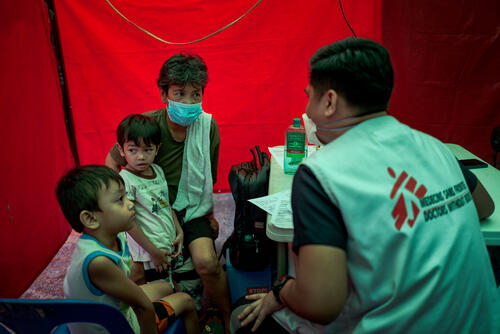
TB PRACTECAL
Translate this page: IsiZulu Қарақалпақша O'zbek, Pусский
TB PRACTECAL is a cutting-edge phase II/III clinical research project to find short, tolerable and effective treatments for people with drug-resistant tuberculosis (DR-TB).
Seizing upon the opportunity of the first new TB drugs in half a century, MSF has partnered with the London School of Hygiene and Tropical Medicine and other global leaders in medical research, as well as ministries of health in affected countries, to take action.
Together, they share one big goal: to save the lives of hundreds of thousands of people with DR-TB and close the deadly treatment gap that is fuelling the global crisis. In order to achieve this, they aim to:
- Identify short, effective and tolerable treatments for people with DR-TB through a clinical trial compliant with international standards for good clinical practice.
- Advocate for global access for DR-TB patients everywhere, through the adoption of a newly identified treatment regimen, if successful, into national protocols and global policy and guidelines.
Initial results show that the new all-oral six-month treatment regimen comprising bedaquiline, pretomanid, linezolid and moxifloxacin (BPaLM) is safer and more effective at treating DR-TB than the currently accepted standard of care. The trial continues to treat and follow up patients until August 2022 with trial close out planned for December 2022.
Update: 22 December 2022
Results published in the New England Journal of Medicine show that the new all-oral six-month treatment regimen comprising bedaquiline, pretomanid, linezolid and moxifloxacin (BPaLM) is safer and more effective at treating DR-TB than the currently accepted standard of care.
Take a look at the TB PRACTECAL trial timeline and results graphic
On 15th December 2022, The WHO released updated guidance for the treatment of DR-TB, now recommending programmatic use of the six-month regimens; BPaLM (Bedaquiline/ Pretomanid/Linezolid/ Moxifloxacin) and BPaL (Bedaquiline/Pretomanid/Linezolid) in people with multi-drug/rifampicin resistant TB (MDR/RR-TB) in place of longer, existing regimens.
Our goal is to respond to the evidence and continue to get these new treatments to people who need them as soon as possible.
In light of the recently updated WHO consolidated guidelines on DR-TB treatment, we aim to provide information on how we used these regimens in the TB-PRACTECAL trial and what it might mean for implementation.
The toolkit contains materials that can be adapted for a number of settings and languages. If you can’t see something suitable here or you would like to know more, please contact us below and we will endeavour to support you.
We hope you find this helpful and will value your feedback no matter what your role is.
Implementation toolkit for BPaLM/BPaL
- Toolkit materials user guide, patient leaflet, booklet and flipbook
FREQUENTLY ASKED QUESTIONS (FAQs)
- About the TB PRACTECAL clinical trial and how it was undertaken
- WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment, 2022 update
- MSF Medical guidelines: Chapter 10: Treatment of multidrug-resistant and rifampicin-resistant tuberculosis
Contact us: tb-practecal@london.msf.org
Since the trial started in January 2017, six sites and one sub-site have opened:
- Nukus, Uzbekistan, January 2017
- Tashkent, Uzbekistan, January 2019
- Minsk, Belarus, December 2017
- Doris Goodwin, KwaZulu-Natal, South Africa, November 2018
- Sub-site: THINK Clinical Trial Unit, Hillcrest (formally Don McKenzie), KwaZulu-Natal, South Africa, October 2018
- Helen Joseph Hospital, Johannesburg, South Africa, December 2019
- King DinuZulu Hospital, KwaZulu-Natal, South Africa, November 2019
Phase II-III clinical research
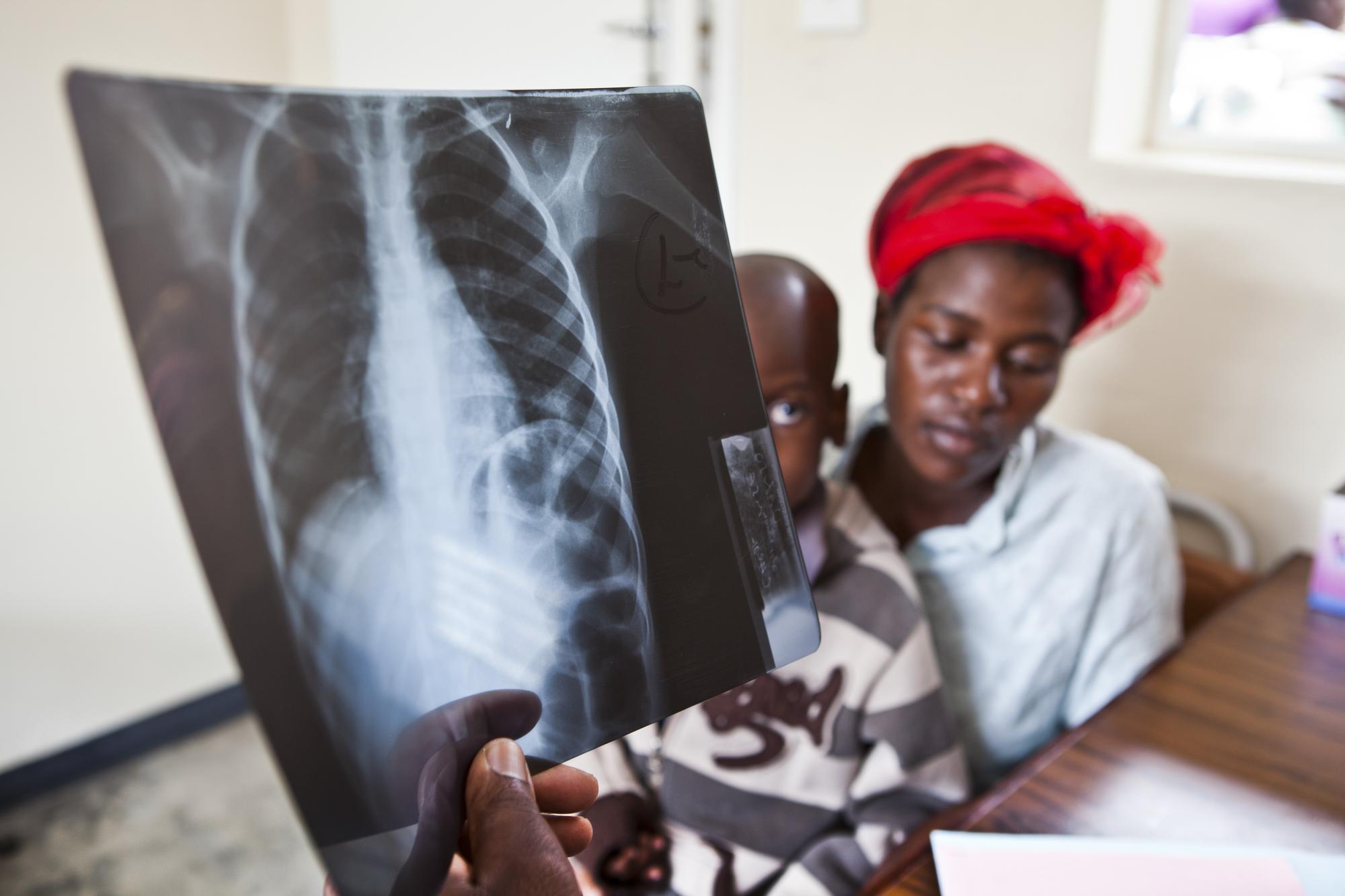
TB PRACTECAL is a multi-centre, multi-arm, open-label, randomised, controlled, phase II-III trial to identify a shortened, safe, effective and tolerable new treatment regimen for adults with pulmonary multidrug-resistant tuberculosis (MDR-TB).
Breaking new ground
Designed to maximise the possibility of finding patient-centred treatments and to speed progress, we intend to:
- Combine two new and highly promising anti-TB drug classes, diarylquinolines and nitroimidazoles, increasing the potential to radically improve treatment
- Identify a six-month regimen to dramatically reduce treatment time
- Utilise a pragmatic and adaptive trial design to speed progress and ensure that a new regimen is relevant for the people who need it most
Stage one of the study identified regimens containing the new drugs bedaquiline and pretomanid for further evaluation based on safety and efficacy outcomes after eight weeks of treatment.
Stage two of the study, which began in November 2020, evaluates the safety and efficacy of the best performing new regimen at 18 months, compared with the World Health Organization recommended standard of care used locally, which makes up the control arm of the trial.
Patient needs and safety lie at the heart of TB PRACTECAL, in full accordance with the Declaration of Helsinki and international standards for good clinical practice.
The new regimens in the study are designed to respond first and foremost to the needs of people with DR-TB – with few pills (no injections), taken orally, for six-months.
It is hoped that patients will experience fewer side effects so that they can comfortably complete their treatment at home.
Furthermore, the regimens should be safe and effective for people living with HIV and co-infected with DR-TB, who are particularly vulnerable.
If successful, a new treatment of this kind could enable low and middle-income countries most affected by the DR-TB epidemic to dramatically increase treatment services. And with treatment still the best form of prevention, this could help to turn the global DR-TB crisis around.
The study incorporates an important community engagement component, with an overarching strategy and country-level plans adapted to each context for optimal relevance.
At its core is the ‘commitment to creating a beneficial, respectful, sustained and transparent partnership that addresses the interests of all stakeholders in the research project, while supporting its ethical and scientifically rigorous conduct’.
To this end, TB PRACTECAL aims to:
- Engage in a dialogue with community stakeholders, sharing knowledge and insights – in order to establish open communications, address local interests, develop mutual understanding and optimise the beneficence and relevance of the research.
- Develop a foundation of understanding and sensitise community stakeholders about research – in order to build on the capacity, knowledge and skills of people to actively engage in an effective partnership role, in support of the study’s ethical and scientifically rigorous conduct.
- Ensure community stakeholders are adequately informed and empowered throughout the research – in order to foster a transparent, respectful and constructive collaboration towards the successful implementation of the study.
As with all clinical trials, there are potential risks associated with using new therapies administered in new combinations.
The trial is overseen by a Data and Safety Monitoring Board, part of a strong governance structure, to ensure that the highest patient safety, scientific and ethical standards are met throughout.
Governance
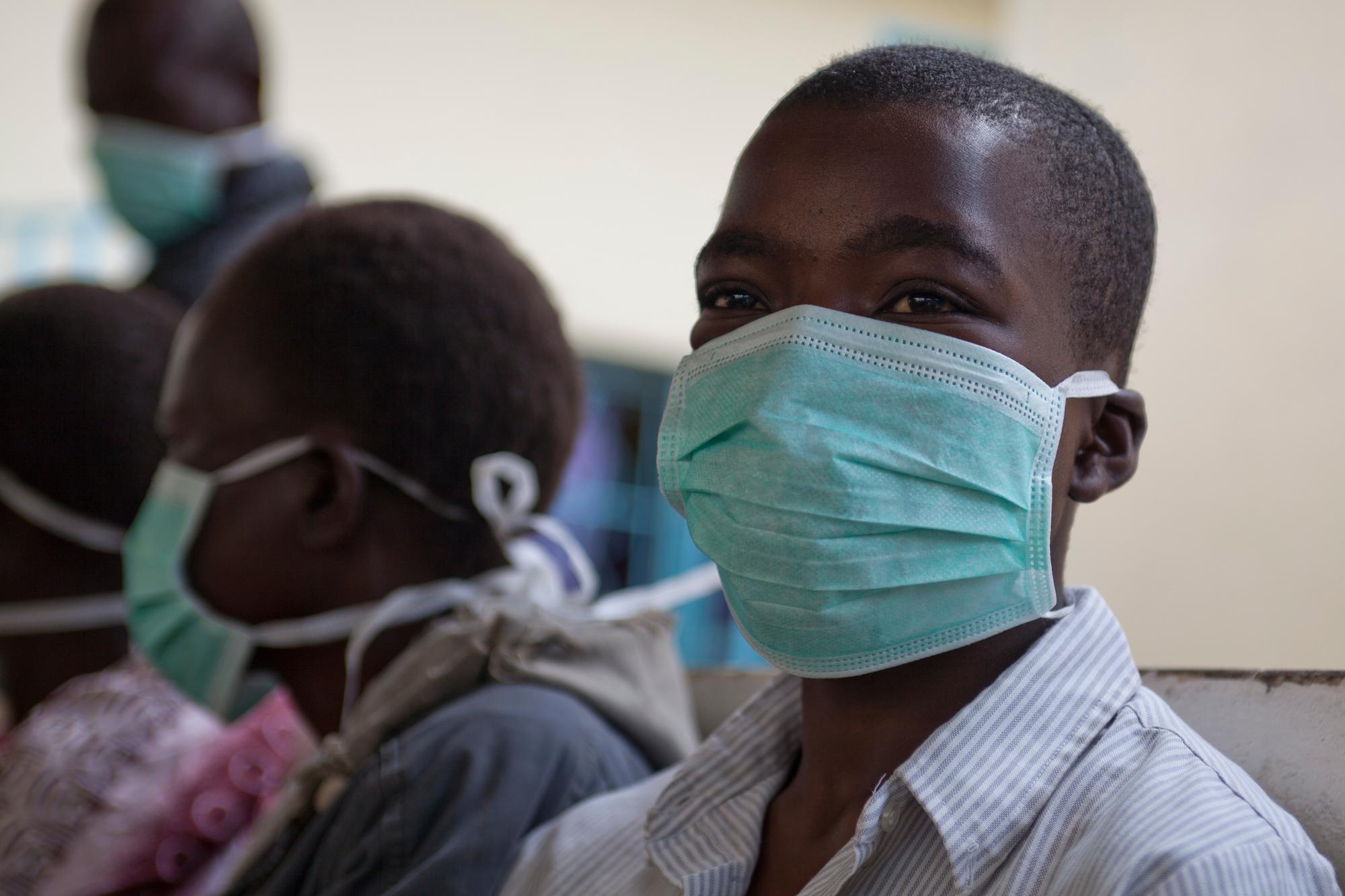
Day-to-day coordination of the TB PRACTECAL study is managed by the Project Management Team, housed in the Manson Unit at MSF UK, a part of the MSF Operation Centre Amsterdam Public Health Department. Ethical and scientific rigour is maintained via a strong governance structure, including a Steering Committee and an external Scientific Advisory Committee consisting of world-renowned experts.
An external and independent Data and Safety Monitoring Board also ensure that the highest standards in patient safety and data reliability are met.
Ethics oversight
Independent Ethics Review Boards (ERBs) are charged with protecting the rights, safety and wellbeing of clinical trial participants.
They review all aspects of a study before deciding whether it can go ahead or not. Before a site is allowed to start enrolling patients, the ERB will review all study-related materials including the protocol, and patient materials. ERB’s also perform periodic reviews throughout the study’s duration.
TB PRACTECAL has been approved by the following independent Ethics Review Boards:
- Médecins Sans Frontières/Doctors Without Borders (MSF) Ethics Review Board
- London School of Hygiene and Tropical Medicine (LSHTM) Observational / Interventions Research Ethics Committee
- Uzbekistan: Ethical Committee under the Ministry of Health of the Republic of Uzbekistan
- Belarus: Ethics Committee of the State Institution Republican Scientific and Practical Centre of Pulmonology and Tuberculosis
- South Africa: Pharma Ethics Independent Research Ethics Committee
- South Africa: University of the Witwatersrand, Johannesburg, Human Research Ethics Committee: (Medical)
Key structures
Bern-Thomas Nyang’wa, Chief Investigator
Bern (MB; BS, MPH [Int]) is a Malawian public health doctor with extensive MDR-TB and international health experience. Dr Nyang’wa worked with MSF between 2004 and 2008 based in Malawi, Nigeria, Chad and the Central African Republic.
Since 2009, he has advised TB programmes as part of the Manson Unit, MSF-UK with a focus on clinical and programmatic management of paediatric and drug-resistant TB in Colombia, Georgia, Abkhazia, Uzbekistan, Tajikistan, Ethiopia and CAR.
He guest lectures at the London School of Health and Tropical Medicine and Nuffield Centre for International Health and Development and is an Honorary Clinical Lecturer at University College London’s Institute for Global Health.
Bern is currently the Medical Director of Operational Centre Amsterdam, MSF.
Emil Kazounis, Project Lead and Clinical Trial Manager
Emil studied medical nutrition and public health and has seven years' experience in clinical research in the UK NHS.
Emil previously set up and managed the UK’s first Blood Cancer Research - Clinical Trial Network and brings the additional experience in managing EU Framework Programme projects and various in situ clinical research programmes in India and Cambodia.
Corinne Merle, Clinical Trialist
Based in the Special Programme for Research and Training in Tropical Diseases at the World Health Organisation (TDR-WHO), Corinne provides technical support for intervention and implementation research projects conducted in Low and Middle-Income Countries.
Corinne is a medical doctor with significant experience as a clinical study coordinator for a phase III TB multicentre registration trial (OFLOTUB).
With over 15 years of international experience as a public health specialist, Corinne’s current work includes Coordinating PI of the RAFA trial (TB/HIV treatment strategies - three parallel arms open labelled study), setting up and mentoring staff to run international multicentre clinical trials to GCP-ICH standards (Benin, Senegal, Guinea).
A trained infectious diseases and public health specialist, she has a long history of lecturing and leading post-graduate study units for clinical trials, Good Clinical Practice, statistics and epidemiology at London School of Hygiene and Tropical Medicine (LSHTM), as well as in other institutions in Europe, Asia and Africa.
Raymond Omollo, Data Management Lead
Raymond has a PhD in Applied Statistics with over 10 years experience in data management and statistics at various levels of clinical research.
He currently heads the Data and statistics centre of Drugs for Neglected Diseases Initiative (DNDi) Africa.
George Mokua Nyangweso, Data Manager
George joins DNDi ARO as a Senior Data Manager for MSF projects.
Most recently before joining DNDi, George worked as a Data Manager for the Kemri-Wellcome Trust Research Programme, Kilifi, Kenya, that involved managing data for multiple research projects ranging from longitudinal, cross-sectional to clinical trials.
George holds a BSc degree in Mathematics and Computer Science from Jomo Kenyatta University and brings on board over eight years of diversified service experiences in health research data management.
Michael Ochieng, Information Systems Manager
Michael is the Information Systems Manager at Drugs for Neglected Diseases initiative (DNDi) Africa Regional Office and is responsible for setting up and managing clinical data management systems.
He recently completed WHO/TDR career development fellowship program at Infectious Diseases Data Observatory (IDDO) based at the University of Oxford.
David Trevino, Logistics support
David studied Architecture in Barcelona and has 22 years of experience in logistics in MSF. He has worked in Georgia, Tanzania, Rwanda, Guatemala, Mexico, Sierra Leone, Kenya, Somalia, CAR, India, Pakistan, Colombia, Albania and Honduras.
He is currently responsible for first-line support logistics to Chad, Russian Federation, Uzbekistan, Tajikistan and Papua New Guinea.
Eric Kwizera, Logistics Support
Eric has a background in biomedical engineering with over 10 years of experience. Prior to his current position as a back-office advisor for biomed and cold chain, he held a flying biomed advisor position for the past 6 years, supporting missions across OCA in medical equipment management. Before joining MSF, Eric worked in Rwanda as a biomed overseeing medical equipment management in government-owned hospitals countrywide.
Marion Coniin, Logistics Support
Marion Conijn has worked as a pharmacist assistant in several pharmacies. Prior to working for MSF, she worked as a Sales Support Product Coordinator at IDA Foundation, a not-for-profit organisation through which she was introduced to MSF, as they have worked intensively together for many years.
Since April 2017 Marion has been part of the Clinical Trial Team as Operational Procurement Officer. She is responsible for order processing, purchasing and shipping of drugs, medical, lab and logistic supplies.
Henry Santana, Laboratory/Biomed Field Support Advisor
Santana joined the Clinical Trial team in March 2019 providing tier 3 technical support. Primarily for the Nukus Lab, he coordinates between manufacturers, lab personnel, supply and the biomedical technicians supporting all aspects of the lab equipment lifecycle.
Katherine Fielding, Lead Statistician/LSHTM PI
A professor in Medical statistics and epidemiology at LSHTM, Katherine is a biostatistician with 20 years of post-doctoral experience including two years at the Centre for Disease Control and Prevention (CDC) as visiting biostatistician and 15 years at LSHTM.
She has a 10-year history supervising PhD students for TB and HIV in biostatistics and epidemiology and been a biostatistician for eight TB-related studies.
Currently, Katherine also sits on Data and Safety Monitoring Boards for three of the major current new TB drugs clinical trials (ReMox, Rifaquin, MAMS trial of four treatment regimens for new smear-positive TB), is lead statistician in other large TB/HIV clinical trials and has an extensive publication history in TB.
Matt Dodd, Statistician
Matt is a Research Fellow based in the Department of Medical Statistics at the London School of Hygiene and Tropical Medicine.
He provides expertise in the data management and statistical analysis of randomised controlled clinical trials, including non-inferiority and cluster randomised trials.
Pascal Jolivet, Clinical Trial Laboratory Coordinator
Pascal's background is in haematology and in various fields of analysis having completed his studies in medical biology. He worked for eight years overseeing laboratory activities at the “Centre Hospitalier Intercommunal” de Quimper, Bretagne, a large hospital in the west of France.
Pascal joined the TB PRACTECAL team in November 2019. Prior to his current post in the Manson Unit, he worked for MSF for four years on controls for communicable diseases (Hep C, TB, HIV, Malaria), bacteriology, blood transfusion and general laboratory (serology, biochemistry, haematology, quality control) as a Field Lab Manager, Quality Control Advisor, and Regional Technical Referent in Mali, Kenya, and the Asia region (covering Cambodia, Bangladesh, PNG, Philippines, and Pakistan), respectively.
Erin da Costa, Clinical Trial Pharmacist
Erin worked as a clinical hospital pharmacist for five years in Canada before pursuing her Masters of Public Health in Developing Countries at the London School of Hygiene and Tropical Medicine (LSHTM).
She joined MSF in 2010 as the Pharmacist Advisor in Amsterdam and was responsible for assuring quality supply and use of pharmaceutical products in all of MSF Holland's missions.
Catherine Berry, Global Principal Investigator
Catherine is an infectious diseases physician (FRACP) with over 12 years of clinical experience in internal medicine. Whilst completing a Masters in Public Health and Tropical Medicine, she joined MSF in 2014 working in Uzbekistan and Jordan. She is a conjoint lecturer with the University of Newcastle.
She has extensive experience in research coordination as well as the care of MDR-TB patients.
Ilaria Motta, Medical Monitor
Ilaria is an infectious diseases physician with experience in managing HIV and TB infections in resource-limited settings.
She obtained a Diploma in Tropical Medicine and Hygiene (DTM&H) in 2011. She has recently completed her PhD project focusing on the pharmacokinetics of TB drugs.
In 2015 she worked for MSF in the Central African Republic as a medical doctor.
Jocelyn Jansen van Vuuren, Medical Monitor Support
Jocelyn joined TB PRACTECAL in November 2020 as a sub-investigator at the THINK Doris Goodwin Hospital site before moving to the UK and joining the MSF team in 2022. She has experience working in emergency departments in various settings. She holds a DipPECSA (Diploma in Primary Emergency Care) and DipHIVManSA (Diploma in HIV management) from the CMSA.
Nathalie Lachenal, Pharmacovigilance Officer
Nathalie has been working in pharmacovigilance in various therapeutic areas (neuro-/immunological diseases, oncology) in the pharmaceutical industry for seven years.
She has set up a pharmacovigilance system for MSF to ensure appropriate detection, monitoring, follow-up and prevention of adverse drug reactions, for the TB PRACTECAL and End-TB projects.
Nicola James, Clinical Research Associate
Nicola joined TB PRACTECAL in February 2020. She has over five years of experience coordinating operational research studies and supporting clinical trials, mainly in Southeast Asia. Her areas of research have focused on drug-resistant malaria and other infectious diseases for organisations including LSHTM, Mahidol-Oxford Tropical Medicine Research Unit and International Organisation for Migration. Nicola holds a Masters in Control of Infectious Diseases, from LSHTM.
Rubin Rose-Key, Clinical Research Associate
Rubin joined the TB PRACTECAL team in March 2022. He has completed a BSc in Adult Nurse and has experience working as a nurse within critical care, as well as in clinical research. His experience in clinical research focussed on viral respiratory studies, along with a government-funded COVID-19 characterisation study. Rubin is also currently carrying out an MSc in Global Health, due to be completed in September 2022.
Charlotte Batts, Clinical Trial Project Administrator
Charlotte joined MSF in August 2020. Before joining the TB PRACTECAL team she worked at the Victoria and Albert Museum providing project support to the two V&A East major capital projects; the Stratford Waterfront Museum and the Collections Research Centre. Prior to that, she was the Executive Assistant to the Director of Finance and Resources and the Digital, Commercial and Exhibitions Director. She has also worked for the Royal National Orthopaedic Hospital Trust supporting the Therapies Department directors and managed several IT and medical records projects. She has a degree in History (BA).
Bern-Thomas Nyang’wa (chair), Medical Director, Operational Centre Amsterdam, MSF
Melissa McRae, former Medical Director, Operational Centre Amsterdam, MSF
David Moore, Professor of Infectious Diseases and Tropical Medicine, LSHTM
Koert Ritmeijer, Health Advisor Coordinator, Operational Centre Amsterdam, MSF
Phil du Cros, Infectious Diseases Specialist, Burnet Institute
Mel Spigelman, President and Chief Executive Officer, TB Alliance
Eugene Sun, Senior Vice President, TB Alliance
Morounfolu Olugbosi, Senior Director, Clinical Development at Global Alliance for TB Drug Development, TB Alliance
Robert Horsburgh (chair), Professor of Epidemiology, Boston University School of Public Health
Eric Nuermberger, Associate Professor of Medicine, Division of Infectious Diseases, Department of Medicine, Johns Hopkins University
Graeme Meintjes, Associate Professor of Medicine and Infectious Diseases, University of Cape Town
Kathleen Eisenach, Professor of Pathology and Microbiology, the University of Arkansas for Medical Sciences
Christian Lienhardt, Global TB Programme, World Health Organisation
Andrew Nunn, Associate Director and Chair of Infections Research Theme, Medical Research Council Clinical Trials Unit (MRC CTU) at University College London
Christoph Lange, Head of Clinical Infectious Diseases, Research Center Borstel, Leibniz Center for Biomedical Research
Michael Hughes (Chair), Professor of Biostatistics, Harvard School of Public Health
Payam Nahid, Professor in Residence at the Division of Pulmonary and Critical Care Medicine (University of California) and Director of the UCSF Center for Tuberculosis. Previously led the WHO Task Force for New TB Drugs and Treatment Regimens.
Johnstone Kumwenda, Professor of Internal Medicine, College of Medicine, University of Malawi
Suman Majumdar, Deputy Program Director for Health Security and co-head of the Tuberculosis Elimination & Implementation Science Group at the Burnet Institute
Todd Lorenz, Clinical Development/Medical Affairs/Scientific Licensing Consultant
International collaboration

As lead investigator and sponsor, the TB PRACTECAL study draws upon vast MSF experience and expertise in DR-TB care and innovation, working in collaboration with Ministries of Health in affected countries, the London School of Hygiene and Tropical Medicine and other global leaders in medical research and innovation.
Latest news: TB PRACTECAL